A system for reporting and reviewing errors is an es-sential component of a medication safety system. Building a safer health system 1999.

Example Of Medication Error Report Form Download Scientific Diagram
ISMP List of Error-Prone Abbreviations Symbols and Dose Designations.
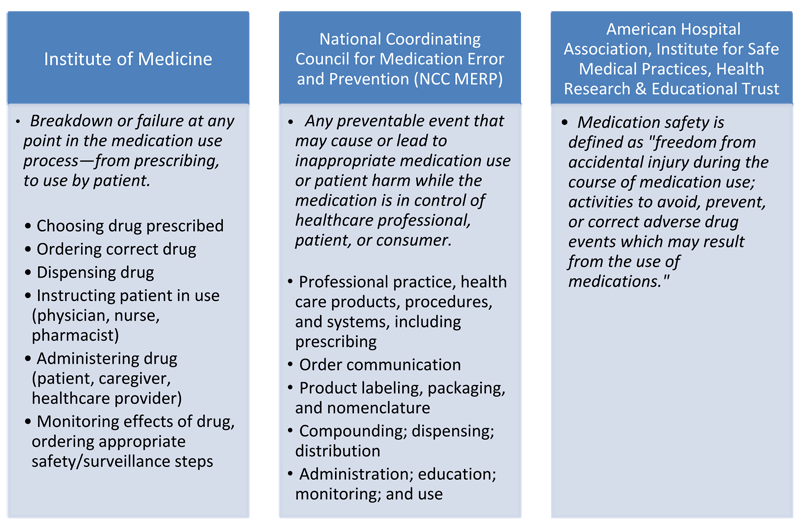
. Medical errors refer to preventable events resulting from healthcare interactions whether these events harm the patient or not. Temporary or permanent impairment in body functions or structures. There is a large and growing body of research addressing medication safety in health care.
From 1983 to 1993 the numbers of deaths from medication errors and adverse reactions to medicines used in US hospitals increased from 2876 to 7391 15 and from 1990 to 2000 the annual number of deaths from medication errors in the UK increased from about 20 to just under 200. To further complicate a practitioners responsibility during patient care there are thousands of health supplements herbs potions and lotions used by the public regularly to treat the. Medical errors are of economic importance and can contribute to serious adverse events for patients.
To optimise the safe use of medicines and reduce avoidable harm to patients. The National Patient Safety Agencys NPSA definition of medication errors is. Developed by the collaborating parties of the Canadian Medication Incident Reporting and Prevention System CMIRPS 2005.
Reporting to MedWatch is easy confidential and secure and it can help save others from being harmed by medication errors. An integrative literature review was guided by Whittemore and Knafls Journal of Advanced Nursing 5 2005 and 546 five-stage review of the 11 articles that met review criteria. Close to 6800 prescription medications and countless over-the-counter drugs are available in the United States.
Includes mental physical sensory functions and pain. A culture of safety encourages nonpunitive reporting of medication errors and near misses. Reports annually associated with a suspected medication error organizations must manage medication properly in order to avoid harming patients.
56 More complete accurate and timely surveillance of medication errors and ADEs will lead to better understanding of the risks and benefits of medication therapies. FDA Adverse Event Reporting System supports the FDAs post-marketing safety surveillance program for all marketed drug and therapeutic biologic products. NAN encourages the sharing and reporting of medication errors so that lessons learned can be used to increase the safety of the medication use system.
With more than 100000 US. We are the first non-profit organization dedicated to the promotion of safe medication practices. This literature covers the extent of the problem of medication errors and adverse drug events the phases of the medication-use process vulnerable to error and the threats all of this poses for patients.
National Reporting and Learning System NRLS - Central database of patient safety incident reports. Research education and advocacy are the foundation of everything we do and our strong collaborative relationships have enabled us to help protect millions of patients. 16 These increases are not surprisingin recent years hospitals have.
Errors and close calls should be reported and analyzed eg root. The FDA enhanced its efforts to reduce medication errors by dedicating more resources to drug safety which included forming a new division on medication errors at the agency in 2002. Institute for Safe Medication Practices ISMP.
Adopt a reporting system. In Kuwait there is a paucity literature detailing the causes forms and risks of medical errors in their state-funded healthcare. The goal is to enhance patient safety and prevent patient harm.
1 As modern healthcare delivery systems continue to evolve emphasis on system design ie. The staff should be encouraged to report without any repercussions. IJCP is a bi-monthly international peer-reviewed journal that publishes original research data new ideas and discussions on a broad range of topics related to clinical pharmacy.
NAN encourages the sharing and reporting of medication errors so that lessons learned can be used. The only way to reduce medication errors is to develop a reporting system and then make changes to prevent similar errors from reoccurring. Adverse events and medication errors are coded to terms in the Medical Dictionary for Regulatory Activities MedDRA terminology.
Medication Errors Policy Version 21 May 2019 4 10 Introduction 11 What is a Medication Error. Even a near miss should be reported. The National Alert Network NAN publishes the alerts from the National Medication Errors Reporting Program.
It also addresses systems factors that contribute to medication errors. It is a great learning experience and enhances safety. As this body of literature is evaluated the fact that there are crucial areas about which.
Therefore four additional rights were proposed to include right documentation actionreason form and response. The National Alert Network NAN publishes the alerts from the National Medication Errors Reporting Program. Error-prone abbreviations symbols and dose designations that are relevant mostly in handwritten communications of medication information are highlighted with a dagger.
To ensure prescribers in GP practices identify and report medication related incidents and near misses via the National Reporting and Learning System NRLS Each practice was required to share at least 4 records with the CCG between April 2017 and March 2018. System-related causes of. Reporting an Adverse Event or.
Nurses clinical reasoning and practices that support medication safety are often invisible when the focus is medication errors avoidance. The informatic structure of the FAERS database adheres to the international safety reporting guidance issued by the International Conference on Harmonisation ICH E2B. Technology clinical workflows has become a priority to complement the medication administration process.
If despite your efforts you have problems with a medication talk with your doctor or pharmacist about whether to report it to MedWatch the Food and Drug Administration safety and adverse event reporting program. To err is human.

Medication Error Reporting System
Error Reporting And Disclosure Patient Safety And Quality Ncbi Bookshelf

Medication Error Reporting System

Example Of Medication Error Report Form Download Scientific Diagram

Medication Incident Report Form Download Scientific Diagram

Module 15 Medication Safety And Error Prevention

Module 15 Medication Safety And Error Prevention

Medication Safety Medication Errors Part I Phcl Ppt Video Online Download

Example Of Medication Error Report Form Download Scientific Diagram

Medication Error Reporting System

Medication Error Reporting System

The 5w S Of Medication Incident Reporting Hospital News
Error Reporting And Disclosure Patient Safety And Quality Ncbi Bookshelf
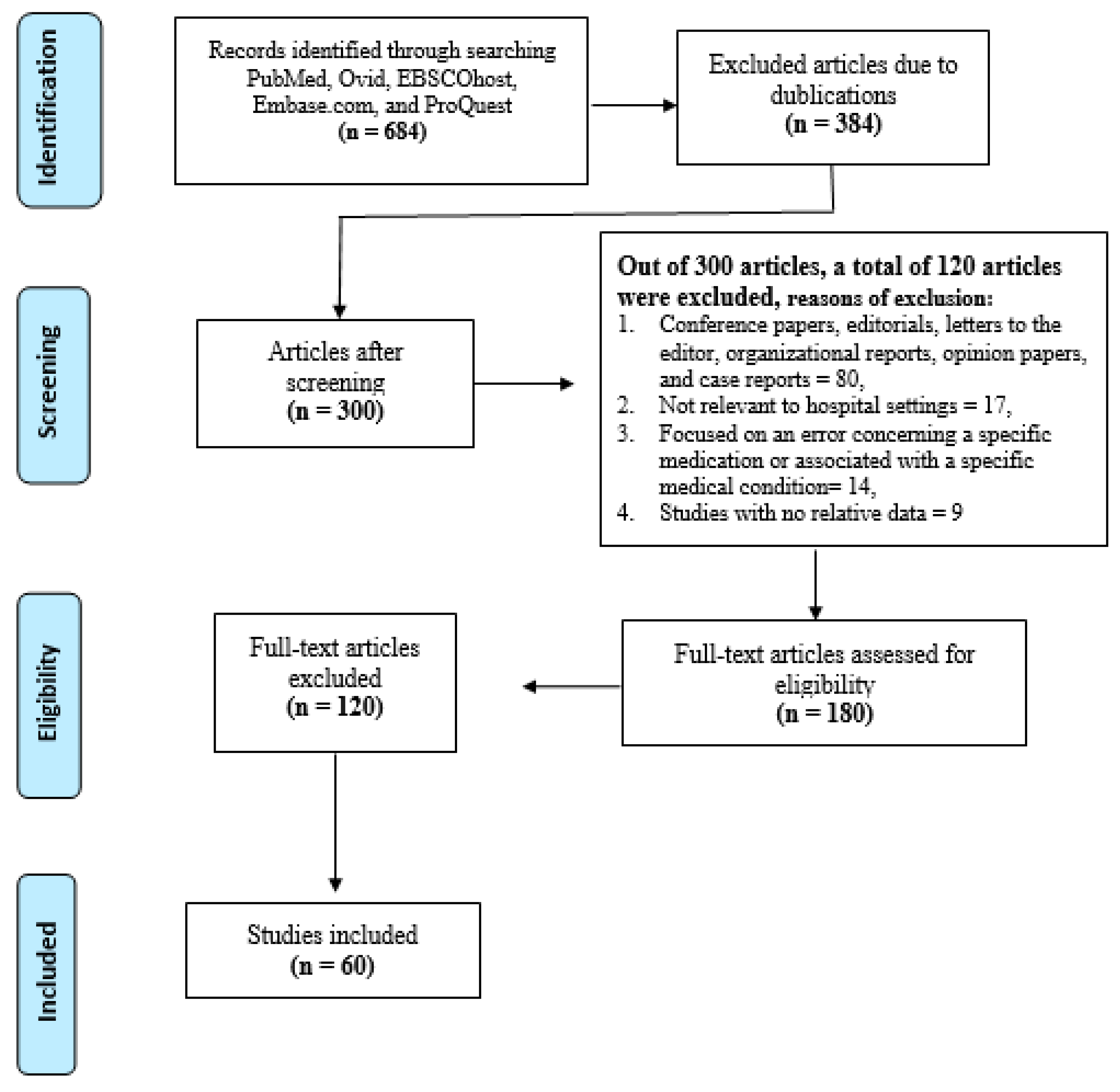
Medicines Free Full Text The Effective Strategies To Avoid Medication Errors And Improving Reporting Systems
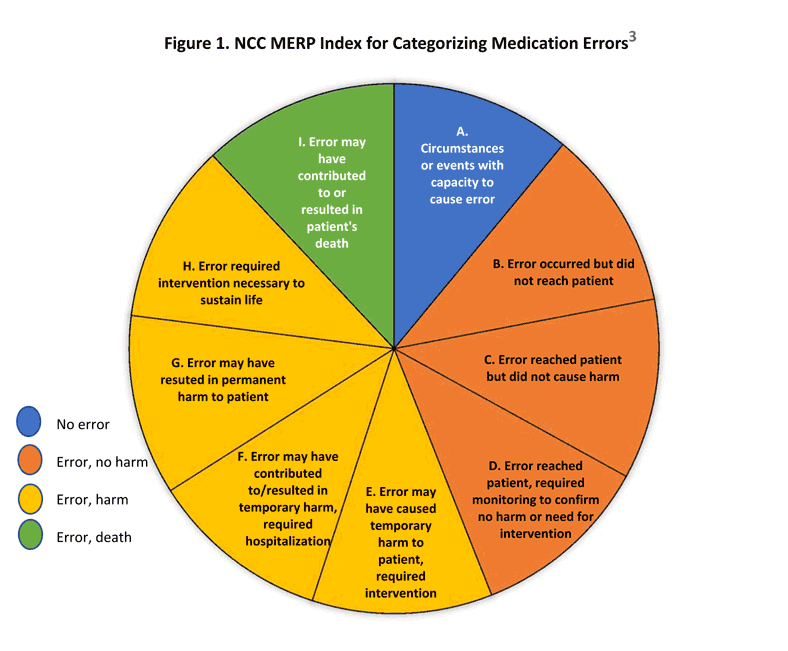
Module 15 Medication Safety And Error Prevention

National Coordinating Council For Medication Error Reporting And Download Scientific Diagram

Medication Error Reporting System

Prevent Medical Errors In Your Practice
